It is suitable for intrathecal injection and other treatment methods (such as systemic analgesics, adjuvant therapy or sheath) Ziconotide is a powerful, selective and reversible N-type voltage-sensitive calcium channel blocker, which is effective for refractory pain, and does not produce drug resistance after long-term administration, and it does not cause physical and mental dependence, nor does it cause life-threatening respiratory depression due to overdose. The recommended daily dose is less, with good curative effect, high safety, less adverse reactions, no drug resistance and addiction. This product has a huge market prospect as a painkiller.



According to incomplete statistics, the incidence of pain in the world is about 35% ~ 45% at present, and the incidence of pain in the elderly is relatively high, about 75% ~ 90%. An American survey shows that the incidence of migraine increased from 23.6 million in 1989 to 28 million in 2001. In the investigation of chronic pain in six cities in China, it is found that the incidence of chronic pain in adults is 40%, and the rate of medical treatment is 35%; The incidence of chronic pain in the elderly is 65% ~ 80%, and the rate of seeing a doctor is 85%. In recent years, the medical expenses for pain relief are increasing year by year.
From 2013 to July 2015, the Pain Research Center in the United States and several medical institutions conducted a long-term, multi-center and observational study on intrathecal injection of ziconotide in 93 adult white female patients with severe chronic pain. The pain digital score scale and the overall sensory score of patients with intrathecal injection of ziconotide and without injection of ziconotide were compared Among them, 51 patients used intrathecal injection of ziconotide, while 42 patients did not. The baseline pain scores were 7.4 and 7.9, respectively. The recommended dose of intrathecal injection of ziconotide was 0.5-2.4 mcg/ day, which was adjusted according to the patient's pain response and side effects. The average initial dose was 1.6 mcg/ day, 3.0 mcg/ day at 6 months and 2.5 at 9 months. At 12 months, it was 1.9 mcg/ day, and after 6 months, the decrease rate was 29.4%, the contrast increase rate was 6.4%, and the improvement rate of overall sensory score was 69.2% and 35.7% respectively. After 12 months, the decrease rate was 34.4% and 3.4% respectively, and the improvement rate of overall sensory score was 85.7% and 71.4% respectively. The highest side effects were nausea (19.6% and 7.1%), hallucination (9.8% and 11.9%) and dizziness (13.7% and 7.1%). The results of this study once again confirmed the effectiveness and safety of ziconotide recommended as a first-line intrathecal injection.
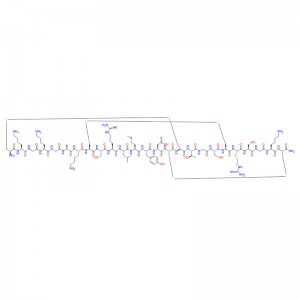
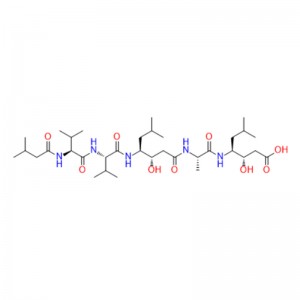
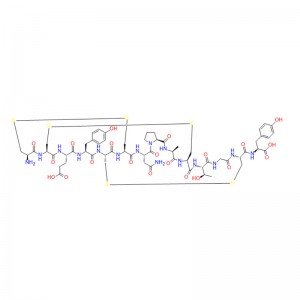
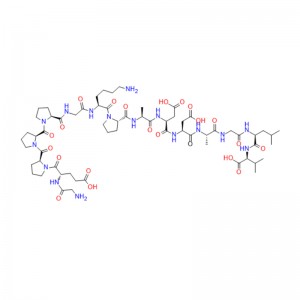
The preliminary study on ziconotide can be traced back to the 1980s, when the potential therapeutic application of rigid and protein-like peptides in conus venom was explored for the first time. These conotoxins are small peptides rich in disulfide bonds, usually 10-40 residues in length, to target various ion channels, GPCR and transporter proteins efficiently and selectively. Ziconotide is a 25-peptide derived from Conus magus, which contains three disulfide bonds, and its short β-fold is spatially arranged into a unique three-dimensional structure, which allows it to selectively inhibit CaV2.2 channels.