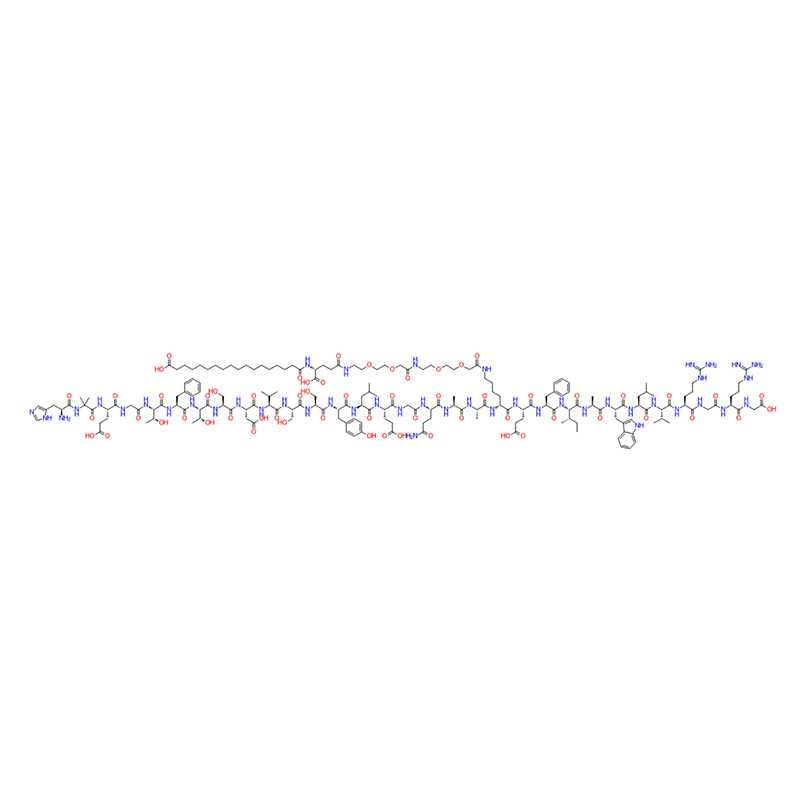
Semaglutide is probably the most effective GLP-1 agonist.
At present, the mainstream weight-loss drugs on the market include orlistat from Roche, liraglutide from Novo Nordisk and semaglutide.
Wegovy, a GLP-1 analogue of Novo Nordisk, was approved by the FDA in 2017 to treat type 2 diabetes. In June 2021, the FDA approved the slimming indication of Wegovy.
In 2022, the first complete commercialization year after the listing of Wegovy, Wegovy gained $877 million in weight loss indications.
With the listing of semaglutide, the subcutaneous administration once a week has greatly improved the compliance of patients, and the weight loss effect is obvious. The weight loss effect in 68 weeks is 12.5% higher than that in placebo (14.9% vs 2.4%), and it has become a star product in the weight loss market for a time.
In the first quarter of 2023, Wegovy achieved a revenue of 670 million US dollars, up 225% year-on-year.
The approval of the weight-loss indication of semaglutide is mainly based on a phase III study called STEP. STEP study mainly evaluates the therapeutic effect of subcutaneous injection of semaglutide 2.4mg once a week compared with placebo on obese patients.



The STEP study included a number of trials, in which about 4,500 overweight or obese adult patients were recruited, including:
STEP 1 study (assisted lifestyle intervention) compared the 68-week safety and efficacy of subcutaneous injection of semaglutide 2.4mg once a week with placebo in 1961 obese or overweight adults.
The results showed that the average change of body weight was 14.9% in the semaglutide group and 2.4% in the PBO group. Compared with PBO, the gastrointestinal side effects of semaglutide are more common, but most of them are transient and can subside without permanently stopping the treatment regimen or prompting patients to withdraw from the study. STEP1 research shows that semaglutide has a good weight loss effect on obese patients.
STEP 2 study (obese patients with type 2 diabetes mellitus) compared the safety and efficacy of subcutaneous injection of semaglutide 2.4 mg once a week with placebo and semaglutide 1.0mg in 1210 obese or overweight adults for 68 weeks.
The results showed that the average body weight estimates of the three treatment groups changed significantly, with -9.6% when using 2.4 mg of semaglutide, -7% when using 1.0mg of semaglutide, and -3.4% when using PBO. STEP2 research shows that semaglutide also shows good weight loss effect for obese patients with type 2 diabetes.
STEP 3 study (adjuvant intensive behavioral therapy) compared the 68-week difference in safety and efficacy between subcutaneous injection of semaglutide 2.4 mg once a week and placebo combined with intensive behavioral therapy in 611 obese or overweight adults.
In the first 8 weeks of the study, all subjects received low-calorie diet replacement diet and intensive behavioral therapy throughout the 68-week program. Participants are also required to do 100 minutes of physical activity every week, with an increase of 25 minutes every four weeks and a maximum of 200 minutes per week.
The results showed that the body weight of patients treated with semaglutide and intensive behavior therapy decreased by 16% compared with baseline, while that of placebo group decreased by 5.7%. From the data of STEP3, we can see the effect of exercise and diet on weight loss, but interestingly, strengthening lifestyle seems to have little effect on strengthening the drug effect of semaglutide.
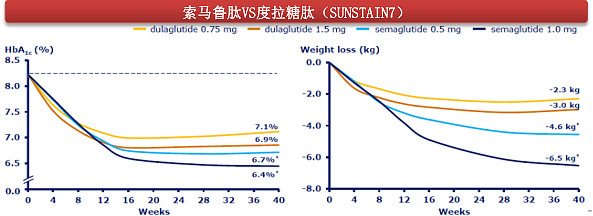
(Comparison of weight loss rate between Semaglutide group and Dulaglutide group)
The drug can increase glucose metabolism by stimulating pancreatic β cells to secrete insulin; And inhibit pancreatic alpha cells from secreting glucagon, thereby reducing fasting and postprandial blood sugar.
(Comparison of body weight between Semaglutide treatment group and placebo)
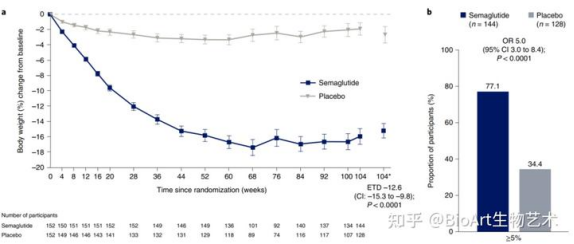
Compared with placebo, Semaglutide can reduce the risk of main composite end points (first cardiovascular death, nonfatal myocardial infarction, nonfatal stroke) by 26%. After 2 years of treatment, Semaglutide can significantly reduce the risk of non-fatal stroke by 39%, non-fatal myocardial infarction by 26% and cardiovascular death by 2%. In addition, it can also reduce food intake by reducing appetite and slowing down the digestion of the stomach, and ultimately reduce body fat, which is conducive to weight loss.
In this study, it was found that phentermine-topiramate and GLP-1 receptor agonist were proved to be the best weight-loss drugs among overweight and obese adults.