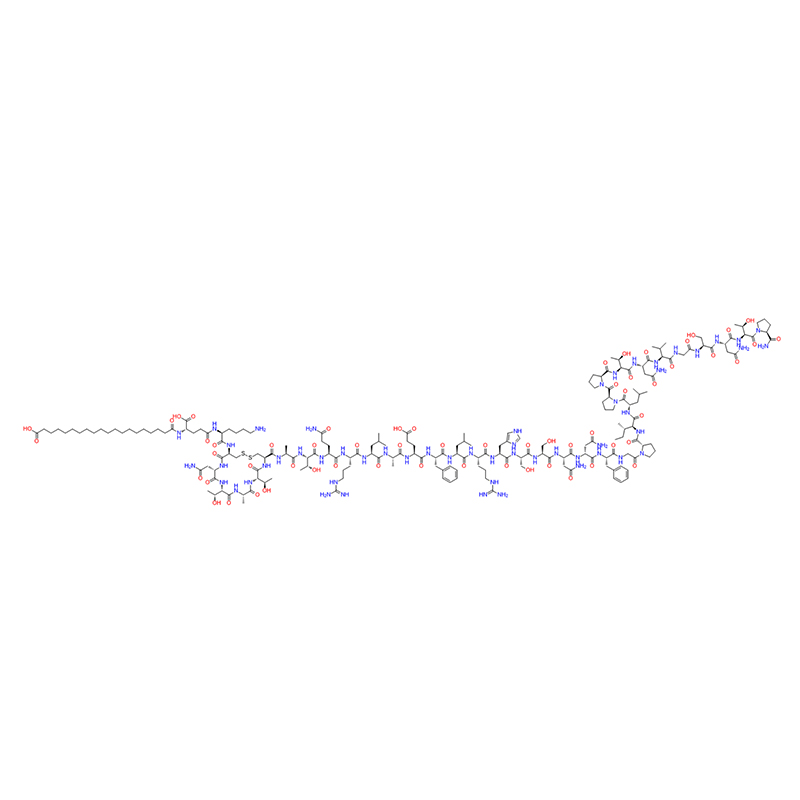
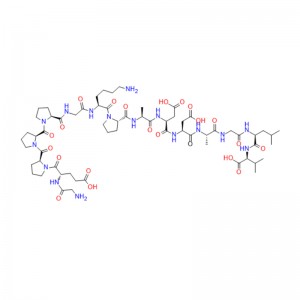
Cagrilintide is a synthetic peptide that mimics the action of amylin, a hormone secreted by the pancreas that regulates blood glucose levels and appetite. It is composed of 38 amino acids and contains a disulfide bond. Cagrilintide binds to both amylin receptors (AMYR) and calcitonin receptors (CTR), which are G protein-coupled receptors expressed in various tissues, such as the brain, the pancreas, and the bone. By activating these receptors, cagrilintide can reduce food intake, lower blood glucose levels, and increase energy expenditure. Cagrilintide has been investigated as a potential treatment for obesity, a metabolic disorder characterized by excess body fat and increased risk of diabetes, cardiovascular disease, and cancer. Cagrilintide has shown promising results in animal studies and clinical trials, demonstrating significant weight loss and improved glycemic control in obese patients with or without type 2 diabetes.




Figure 1. Homology model of cagrilintide (23) bound to AMY3R. (A) N-terminal part of 23 (blue) is formed by an amphipathic a-helix, deeply buried in the TM domain of AMY3R, while the C-terminal part is predicted to adopt an extended conformation that binds the extracellular part of the receptor. (29,30) The fatty acid attached to the N-terminus of 23, proline residues (which minimize fibrillation), and the C-terminal amide (essential for receptor binding) are highlighted in stick representations. AMY3R is formed by CTR (gray) bound to RAMP3 (receptor-activity modifying protein 3; orange). The structural model was created using the following template structures: a complex structure of CGRP (calcitonin receptor-like receptor; pdb code 6E3Y) and an apo crystal structure of the 23 backbone (pdb code 7BG0). (B) Zoom up of 23 highlighting the N-terminal disulfide bond, an internal salt bridge between residue 14 and 17, a “leucine zipper motif,” and an internal hydrogen bond between residues 4 and 11. (adapted from Kruse T, Hansen JL, Dahl K, Schäffer L, Sensfuss U, Poulsen C, Schlein M, Hansen AMK, Jeppesen CB, Dornonville de la Cour C, Clausen TR, Johansson E, Fulle S, Skyggebjerg RB, Raun K. Development of Cagrilintide, a Long-Acting Amylin Analogue. J Med Chem. 2021 Aug 12;64(15):11183-11194.)
Some of the biological applications of cagrilintide are:
Cagrilintide can modulate the activity of neurons in the hypothalamus, the brain region that controls appetite and energy balance (Lutz et al., 2015, Front Endocrinol (Lausanne)). Cagrilintide can inhibit the firing of orexigenic neurons, which stimulate hunger, and activate anorexigenic neurons, which suppress hunger. For example, cagrilintide can reduce the expression of neuropeptide Y (NPY) and agouti-related peptide (AgRP), two potent orexigenic peptides, and increase the expression of proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART), two anorexigenic peptides, in the arcuate nucleus of the hypothalamus (Roth et al., 2018, Physiol Behav). Cagrilintide can also enhance the satiating effect of leptin, a hormone that signals the body’s energy status. Leptin is secreted by adipose tissue and binds to leptin receptors on hypothalamic neurons, inhibiting orexigenic neurons and activating anorexigenic neurons. Cagrilintide can increase the sensitivity of leptin receptors and potentiate leptin-induced activation of signal transducer and activator of transcription 3 (STAT3), a transcription factor that mediates leptin’s effects on gene expression (Lutz et al., 2015, Front Endocrinol (Lausanne)). These effects can reduce food intake and increase energy expenditure, leading to weight loss.

Figure 2. Food intake in rats after subcutaneous administration of Cagrilintide 23. (adapted from Kruse T, Hansen JL, Dahl K, Schäffer L, Sensfuss U, Poulsen C, Schlein M, Hansen AMK, Jeppesen CB, Dornonville de la Cour C, Clausen TR, Johansson E, Fulle S, Skyggebjerg RB, Raun K. Development of Cagrilintide, a Long-Acting Amylin Analogue. J Med Chem. 2021 Aug 12;64(15):11183-11194.)
Cagrilintide can regulate the secretion of insulin and glucagon, two hormones that control blood glucose levels. Cagrilintide can inhibit glucagon secretion from alpha cells in the pancreas, which prevents excessive glucose production by the liver. Glucagon is a hormone that stimulates the breakdown of glycogen and the synthesis of glucose in the liver, raising blood glucose levels. Cagrilintide can suppress glucagon secretion by binding to amylin receptors and calcitonin receptors on alpha cells, which are coupled to inhibitory G proteins that reduce cyclic adenosine monophosphate (cAMP) levels and calcium influx. Cagrilintide can also potentiate insulin secretion from beta cells in the pancreas, which enhances glucose uptake by the muscles and adipose tissue. Insulin is a hormone that promotes the storage of glucose as glycogen in the liver and muscles, and the conversion of glucose to fatty acids in adipose tissue, lowering blood glucose levels. Cagrilintide can enhance insulin secretion by binding to amylin receptors and calcitonin receptors on beta cells, which are coupled to stimulatory G proteins that increase cAMP levels and calcium influx. These effects can lower blood glucose levels and improve insulin sensitivity, which can prevent or treat type 2 diabetes (Kruse et al., 2021, J Med Chem; Dehestani et al., 2021, J Obes Metab Syndr.).
Cagrilintide can also affect the function of osteoblasts and osteoclasts, two types of cells that are involved in bone formation and resorption. Osteoblasts are responsible for producing new bone matrix, while osteoclasts are responsible for breaking down old bone matrix. The balance between osteoblasts and osteoclasts determines the bone mass and strength. Cagrilintide can stimulate osteoblast differentiation and activity, which increases bone formation. Cagrilintide can bind to amylin receptors and calcitonin receptors on osteoblasts, which activate intracellular signaling pathways that promote osteoblast proliferation, survival, and matrix synthesis (Cornish et al., 1996, Biochem Biophys Res Commun. ). Cagrilintide can also increase the expression of osteocalcin, a marker of osteoblast maturation and function (Cornish et al., 1996, Biochem Biophys Res Commun.). Cagrilintide can also inhibit osteoclast differentiation and activity, which decreases bone resorption. Cagrilintide can bind to amylin receptors and calcitonin receptors on osteoclast precursors, which inhibit their fusion into mature osteoclasts (Cornish et al., 2015). Cagrilintide can also reduce the expression of tartrate-resistant acid phosphatase (TRAP), a marker of osteoclast activity and bone resorption (Cornish et al., 2015, Bonekey Rep.). These effects can improve bone mineral density and prevent or treat osteoporosis, a condition characterized by low bone mass and increased fracture risk (Kruse et al., 2021; Dehestani et al., 2021, J Obes Metab Syndr.)