On July 5, Novo Nordisk launched a phase III clinical trial of CagriSema injection in China, the purpose of which is to compare the safety and efficacy of CagriSema injection with semeglutide in obese and overweight patients in China.
CagriSema injection is a long-acting combination therapy under development by Novo Nordisk, the main components are GLP-1 (glucagon-like peptide-1) receptor agonist smeglutide and a long-acting amylin analog cagrilintide. CagriSema injection can be administered subcutaneously once a week.
The primary objective was to compare CagriSema (2.4 mg/2.4 mg) with semeglutide or placebo once weekly subcutaneously. Novo Nordisk has announced the results of a trial of CagriSema for the treatment of stage 2 diabetes, which proved that CagriSema's hypoglycemic effect is better than that of semeglutide, and nearly 90% of subjects have achieved the HbA1c goal.
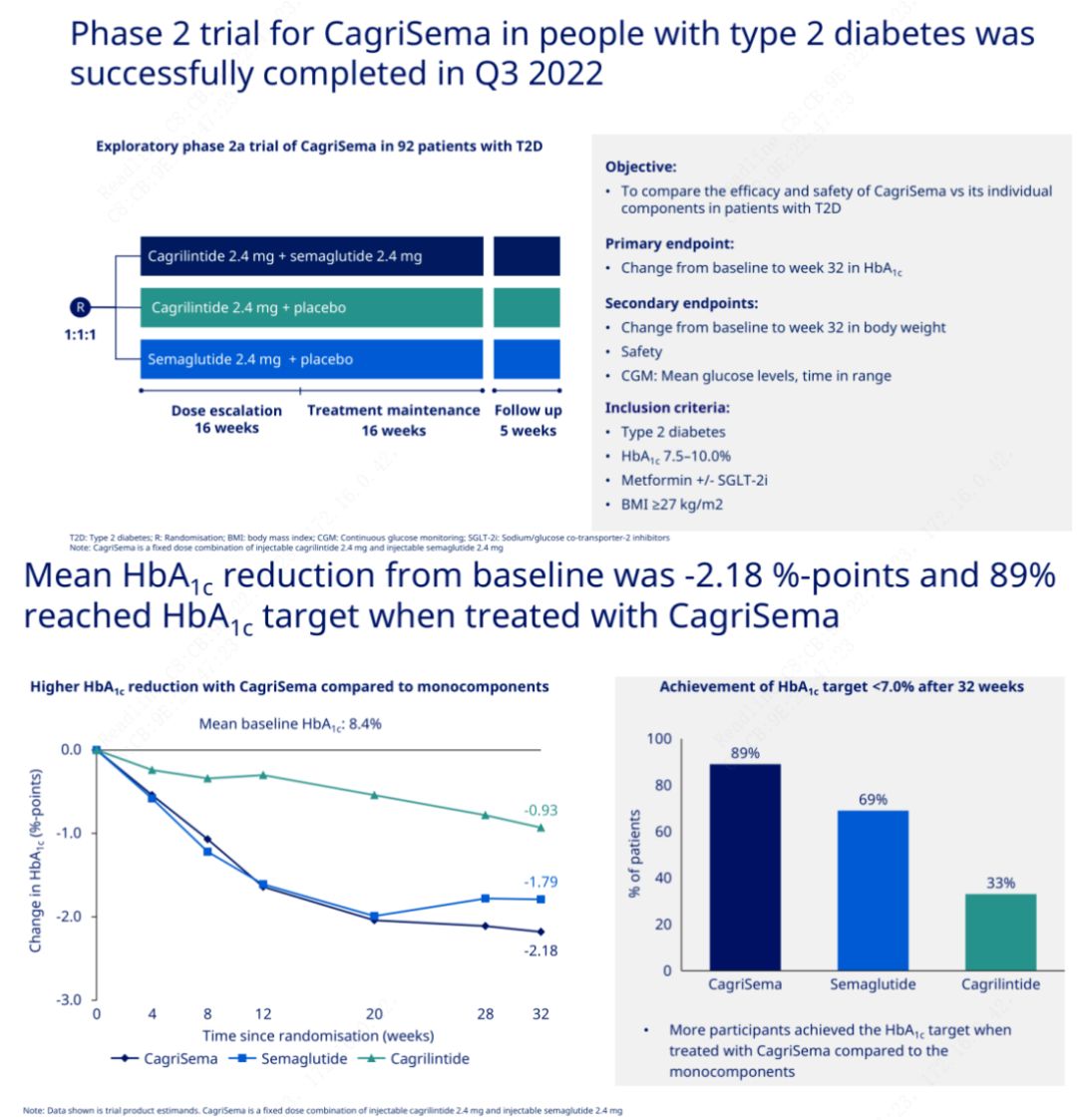
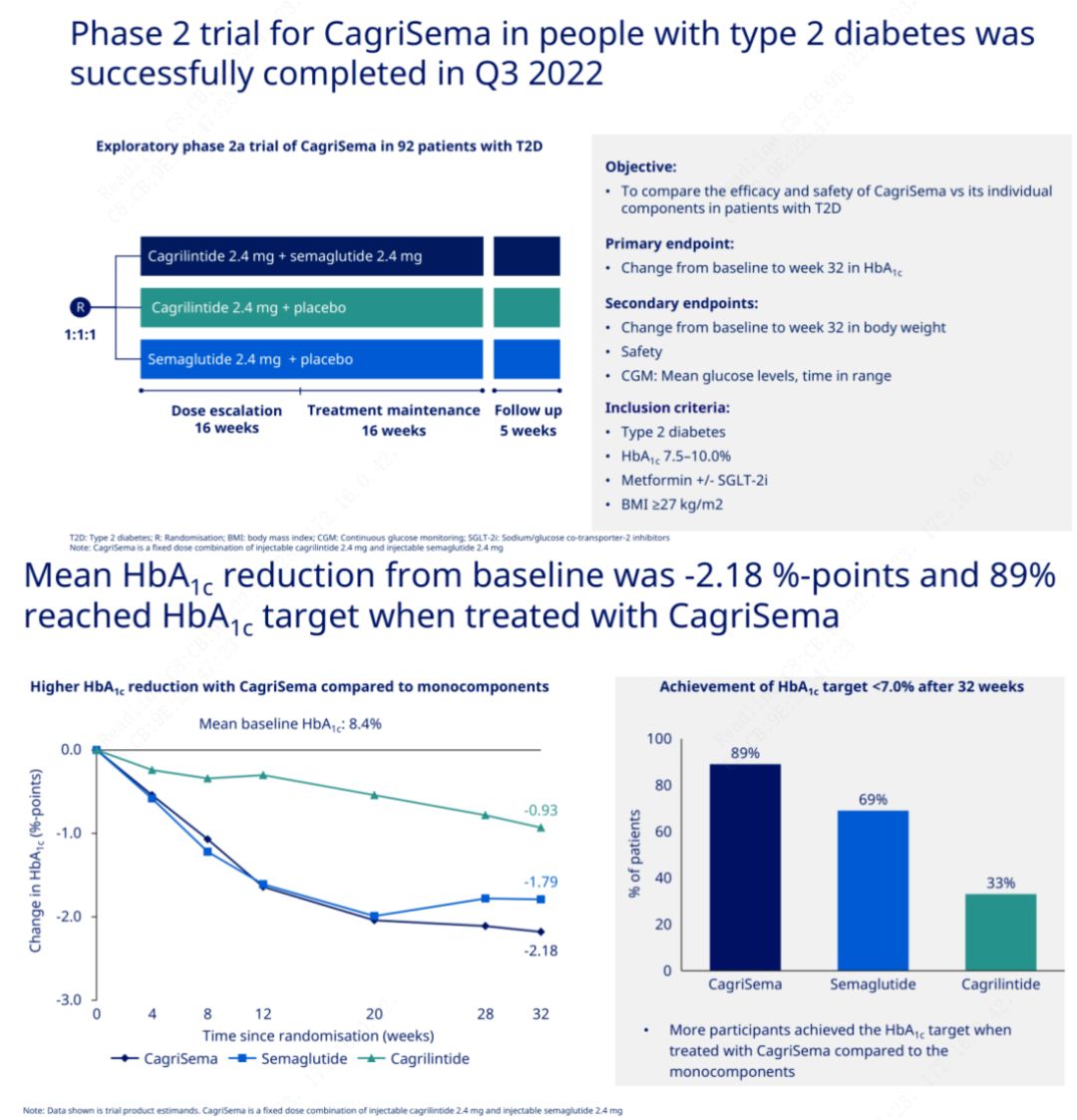
The data showed that in addition to the significant hypoglycemic effect, in terms of weight loss, CagriSema injection significantly outperformed semeglutide (5.1%) and cagrilintide (8.1%) with a weight loss of 15.6%.
The innovative drug Tirzepatide is the world's first approved weekly GIP/GLP-1 receptor agonist. It combines the effects of two incretins into a single molecule that is injected once a week and is a new class of treatments for type 2 diabetes. Tirzepatide was approved by the U.S. Food and Drug Administration (FDA) in May 2022 to improve glycemic control (on a dietary basis and exercise) in adults with type 2 diabetes and is currently approved in the European Union, Japan and other countries.
On July 5, Eli Lilly announced a phase III SURPASS-CN-MONO study on the drug clinical trial registration and information disclosure platform for the treatment of type 2 diabetes patients. SURPASS-CN-MONO is a randomized, double-blind, placebo-controlled phase III study designed to evaluate the efficacy and safety of tirzepatide monotherapy compared to placebo in people with type 2 diabetes. The study planned to include 200 patients with type 2 diabetes who were not on any antidiabetic drugs in the 90 days prior to Visit 1 (except in certain clinical situations, such as acute illness, hospitalization, or elective surgery, short-term (≤14 days) use of insulin).
Type 2 diabetes is expected to be approved this year
Last month, the results of a SURPASS-AP-Combo study were published May 25 in the blockbuster journal Nature Medicine. The results showed that compared with insulin glargine, Tirzepatide showed better HbA1c and weight reduction in the population of type 2 diabetes patients in the Asia-Pacific region (mainly China): HbA1c reduction of up to 2.49% and weight reduction of up to 7.2 kg (9.4%) at 40 weeks of treatment, significant improvement in blood lipids and blood pressure, and overall safety and tolerability were good.
The Phase 3 clinical trial of SURPASS-AP-Combo is Tirzepatide's first study conducted mainly in Chinese patients with type 2 diabetes, led by Professor Ji Linong of Peking University People's Hospital. SURPASS-AP-Combo is consistent with the results of the global SURPASS series of research, which further proves that the pathophysiology of diabetes in Chinese patients is consistent with that of global patients, which is the basis for the simultaneous research and development of new drugs in China and the world, and also provides solid evidence support for giving Chinese patients the opportunity to use the latest diabetes treatment drugs and their clinical application in China as soon as possible.
Post time: Sep-18-2023

